
Methyl Iodide also known as Iodomethane is a chemical compound made up of Methane and iodide – a derivative of Iodine. ‘MeI’ is used as an abbreviation for Methyl iodide and has a molecular formula CH3I. It is a volatile, dense and colourless liquid. It is emitted in small quantities naturally by rice plants. It is majorly produces throughout the world from kelp and algae that are found in the temperate oceans. The amount of methyl Iodide produced every year is estimated to be more than 215 tonnes. It is also produced through bacterial and fungi found on land, although quantity of methyl iodide produced through this process is lesser in amount. Methyl Iodide manufacturer in India recommends that the compound be stored in dark amber to prevent degradation.
UsesOne of the most important application of methyl iodide is that it is used in pesticide as a pre-plant biocide that helps in controlling soil borne pathogens, parasitic nematodes, weeds, and insects.
Here are some other main applications of Methyl Iodide.- It is used in the preparation of Methyl Magnesium Iodide (Grignard reagent).
- It is used as methylating agent in the methylation process during the organic synthesis of carboxylic acid like phenols.
- It is used to prepare Tetra Methyl Ammonium Hydroxide.
| Specification | ||
| HSN No. : 28276090 | CAS No. : 74-88-4 | EC No. : 200-819-5 |
| Molecular Weight : 141.94 g·mol−1 | Molecular Formula : CH3I | |
| Structural Formula | 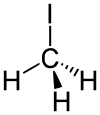 | |
| Appearance | Colorless liquid | |
| Density | 2.28 g mL−1 | |
| Melting Point | −66.5 °C (Predicted) | |
| Solubility in Water | 14 g L−1 (20 °C) | |
| Also known as | Iodomethane, Iodmethan, Iodure de methyle, Joodmethaan | |


